1) Welcome to a new #accredited #tweetorial on a novel agent called a #DEARA–it's #sparsentan, a first-in-class, orally active, single molecule that functions as a high affinity dual-acting antagonist of both endothelin type A (ETA) and angiotensin II subtype 1 (AT1) receptors.
2) ICYMI, "DEARA" means dual endothelin angiotensin receptor antagonist, and these receptors are associated with kidney disease progression. Our expert author is Hector Madariaga @HecmagsMD of TeamKidney @LaheyHospital @LaheyTxpHPB and @KidneyMed #SoMe Editor

3) This educational program is supported by grants from Travere, Bayer, & Otsuka, and is intended for healthcare providers. Faculty disclosures can be found at https://ckd-ce.com/disclosures/. Past programs, still available for CE/#CME credit, are at http://ckd-ce.com.
4) So, as it is 🥇in class, we should start off with a bit of history on how sparsentan was developed.

5) Dr. Robert F. Furchgott (1916–2009) started to do research in the late 1970s on vascular endothelial cells at the State University of New York Downstate in New York City. He must've done well: subsequently he was awarded the #NobelPrize in Physiology or Medicine in 1998.
6) Dr. Furchgott accidentally found that endothelial cells modulate vascular tone by releasing a short-lived vasodilator factor, a labile gas later identified as nitric oxide (#NO). See 🔓
7) Masashi Yanagisawa, with Hiroki Kurihara, became interested in the vasoconstrictor activity of a newly isolated EDCF (endothelium-derived contracting factor); they realized the molecular identity had not been reported, becoming the subject of his thesis
#histmed

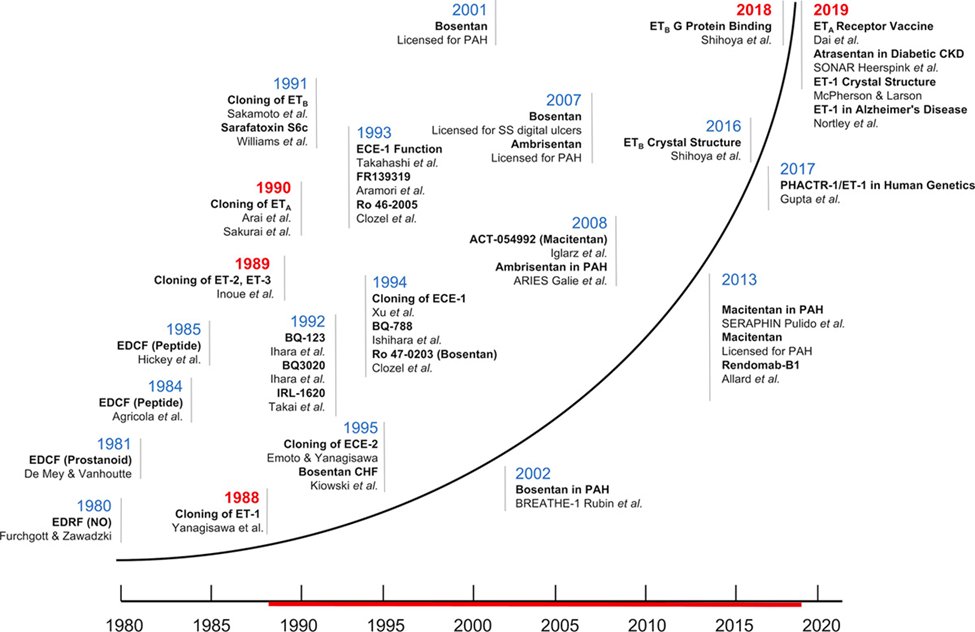
8) Here's a chronology of main discoveries in the endothelin field over the last 30 years 🔓
9) Subsequently, recombinant ET-1 was synthesized and its vasoconstrictor activity demonstrated in vitro and in vivo; the manuscript was published in Nature in 1987.
10) In this video, Dr. Masashi Yanagisawa at The Thirteenth International Conference on Endothelin in Tokyo, Japan, September 2013, talks about his thesis work:
https://vimeo.com/95723638
#histmed
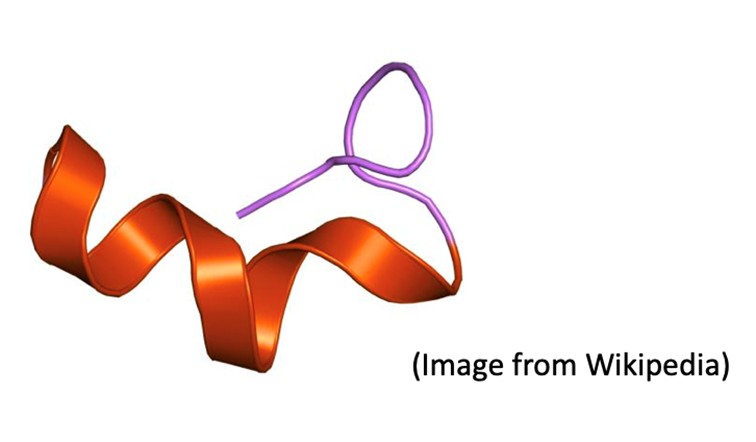
11) ET-1, the main member of the endothelin peptide family, is a 21-amino-acid peptide with a hydrophobic C terminus and 2 cysteine bridges at the N terminus.
12) The endogenous #peptides #angiotensin II (Ang-II) and #endothelin I (ET-1) are powerful vasoconstrictors and mitogens, and both peptides have been implicated in the pathogenesis of #hypertension and #cardiovascular disease
13) Angiotensin II stimulates the production & action of endothelin; endothelin increases the production and function of angiotensin II, thus creating a positive feedback loop. 🔓
14) The endothelin-1 and angiotensin II signaling pathways amplify #kidney injury through multiple mechanisms
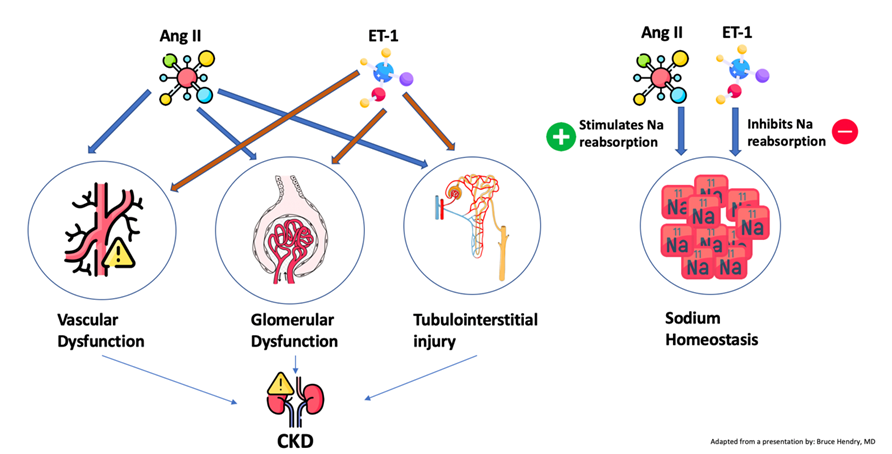
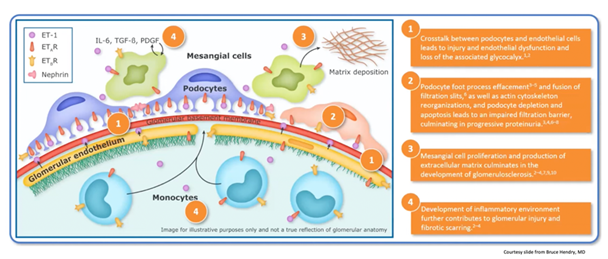
15) ET-1 mediates structural changes that result in #proteinuria due to increased #glomerular filtration barrier permeability
16) Simultaneous antagonism of both the renin-angiotensin system (mediated by angiotensin II via AT1 receptors) & the endothelin system (mediated by ET-1 via ETA receptors) can produce a greater reduction in blood pressure & added CV benefit than antagonizing either system alone
17) This opened the field to a new class of agents: Endothelin Receptor Antagonists (#ERAs), Dual Endothelin Receptor/Angiotensin receptor antagonist (#DEARAs), selective #ETB agonists, receptor-targeting antibodies, & immunizations against #ETA receptors
18) One of these therapeutic agents is Sparsentan (BMS-364567/RE-021), a highly potent dual angiotensin II and endothelin A receptor antagonist with Kis of 0.8 and 9.3 nM, respectively
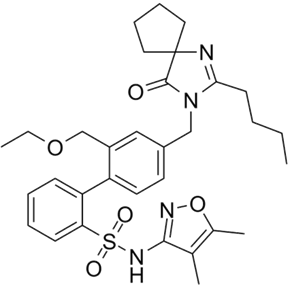
19) Sparsentan is currently being tested in phase 3 clinical trials for the treatment of proteinuric renal diseases such as IgA nephropathy #IgAN and focal segmental glomerulosclerosis #FSGS. See prior programs for both, & earn credit, at
20) First, let’s talk about #IgAN, the most common primary glomerulonephritis in the world. It is more common in individuals from East Asia & White individuals. It occurs rarely in Black individuals. Prevalence is difficult to establish
@IgAN_JBarratt @edgarvlermamd @nephondemand
21) Multihit hypothesis in IgAN: this model integrates findings from studies of galactose-deficient IgA1, anti-glycan response, formation and deposition of IgA1-containing immune complexes, and mechanisms of immune complex-mediated tissue injury
🔓
22) So whaddya know? The culprit in IgAN is Gd-IgA1, which is:
23) Mark your response and RETURN TOMORROW for more education on #DEARA. You are earning 🆓 CE/#CME #physicians #pharmacists #nurses #physicianassociate ! @NWiegley @GarySingerMD @kkalra_22 @ghobby @priti899 @drjamesburton @sibgokcay @NamrataYParikh @NephroShah @croncoIRRIV
24) Welcome back! You are learning from @HecmagsMD about #DEARA: dual endothelin angiotensin receptor antagonist, and you are earning CE/#CME on @ckd_ce, your ONLY source for #accredited #tweetorials! FOLLOW US, @Hmzrage @sarah_m_moran @myadla @SmeetaSinha @kidneydrpanama
25) Did you answer yesterday's quiz? If not, scroll back ⤴️to tweet 22 and COMMIT!
Waiting . . .
Waiting . . .
Waiting . . .
OK, the answer is A: Gd-IgA1 is an autoantigen.
How do we diagnose IgA nephropathy?

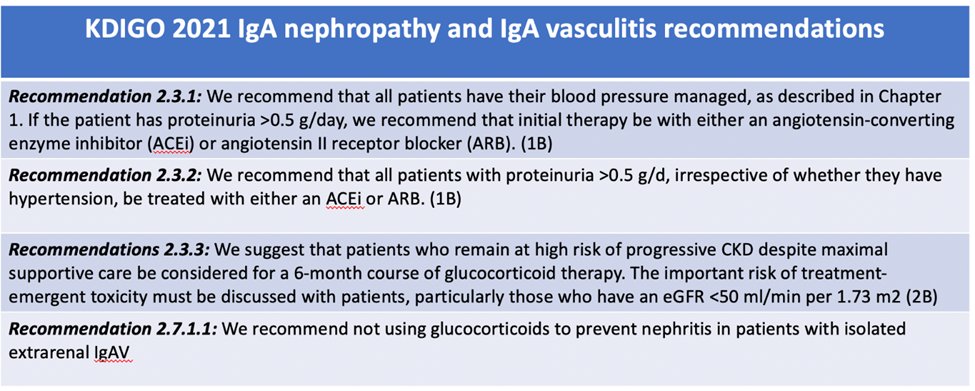
26) IgA treatment, as per @goKDIGO Guidelines, is summarized here. You can learn more (and earn YES EVEN MORE CE/#CME credit) on those guidelines courtesy of @edgarvlermamd at
27) Most recently, TESTING showed that a 6-9 month course of oral methylprednisolone (0.4mg /kg/day with a maximal dose of 32mg/day) ⬇️the risk of major kidney outcomes by 47% and kidney failure by 41%
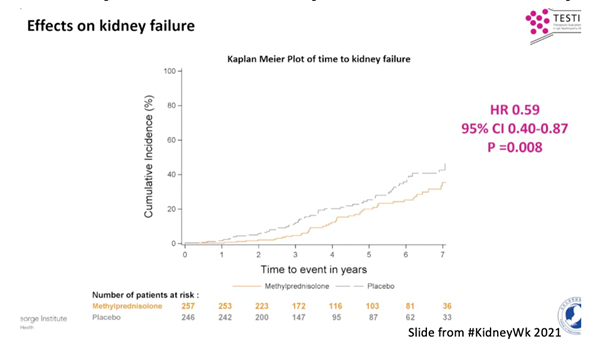
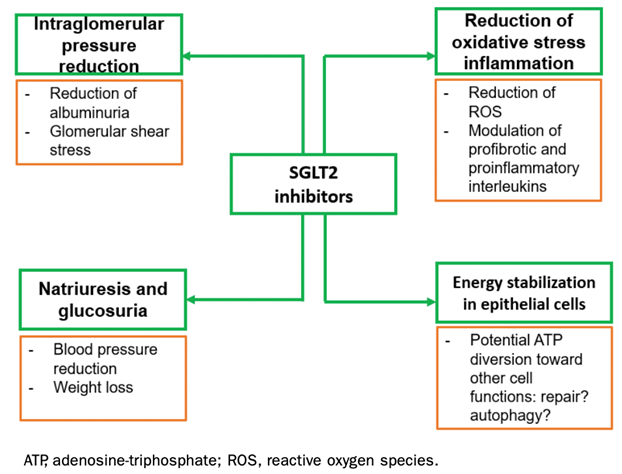
28) Furthermore, #SGLT2i (#FLOZINATE!) might have a role in the treatment of #IgAN
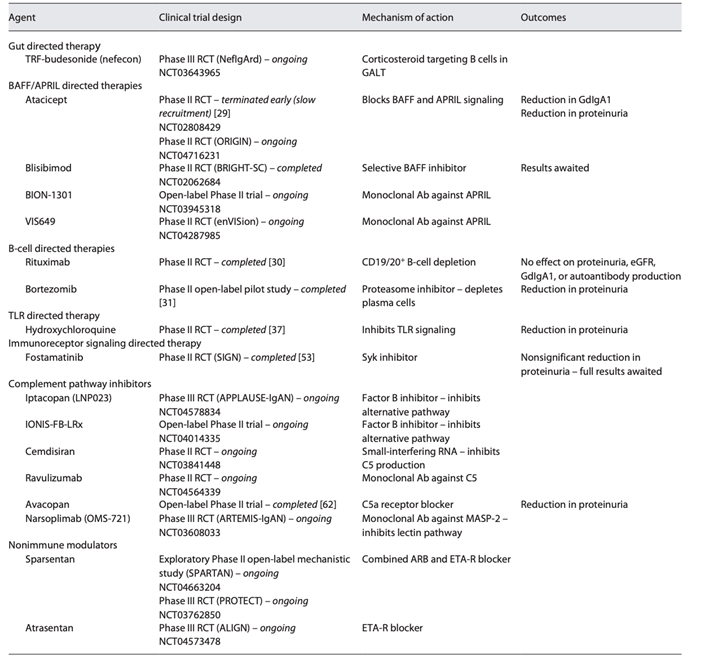
29) Other clinical trials undergoing in the #IgAN field include: #NEFIGARD, ARTEMIS-IGAN, APPLAUSE-IgAN, #ALIGN, BRIGHT.
See 🔓https://www.karger.com/Article/Pdf/519973.
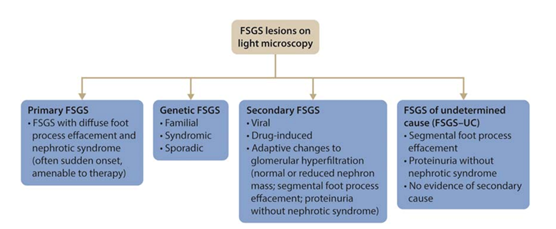
30) Now, let’s turn our attention to #FSGS, a histologic lesion (rather than a specific disease entity) which can cause nephrotic syndrome in children & adults, characterized by the presence of partial sclerosis/segmental of a least one glomerulus (focal) in a kidney biopsy.
31) The lesion of #FSGS can be classified into primary, secondary, genetic and unknown forms. See 🔓https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-Guideline-2021-English.pdf
AND . . . OF COURSE . . . you can learn MORE about #FSGS and earn MORE CE/#CME at https://ckd-ce.com/fsgs1/ &
32) Pts w/primary FSGS present w/peripheral edema, #hypoalbuminemia, & nephrotic range #proteinuria (>3.5 mg/day). In secondary FSGS, non-nephrotic range proteinuria onset is insidious, there is sometimes edema & there is renal impairment over time. See 🔓https://pubmed.ncbi.nlm.nih.gov/24589721/.
33) #suPAR is a myeloid cell-derived circulating factor, which connects innate-immune function to maintenance of the slit diaphragm, activating podocyte αvβ3 integrin.
34) Its activation leads to podocyte foot process effacement, proteinuria, glomerular damage & kidney dysfunction🔓
35) #FSGS lesions are caused by a continuous and sustained injury to podocytes, leading to glomerulosclerosis and persistent proteinuria
🔓
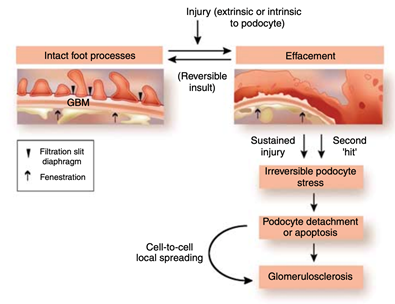
36) In primary FSGS, spontaneous remission is rare, and in 50% of the cases (untreated or unresponsive) progress to #ESKD in 6-8 years. It recurs in one-third of #kidneytransplant recipients.
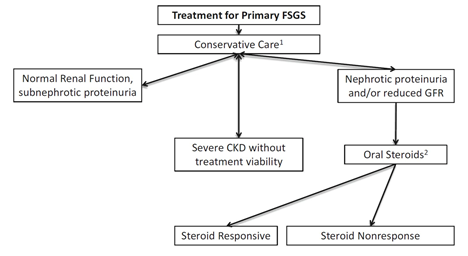
37) Here are the @goKDIGO recommendations for the treatment of #FSGS
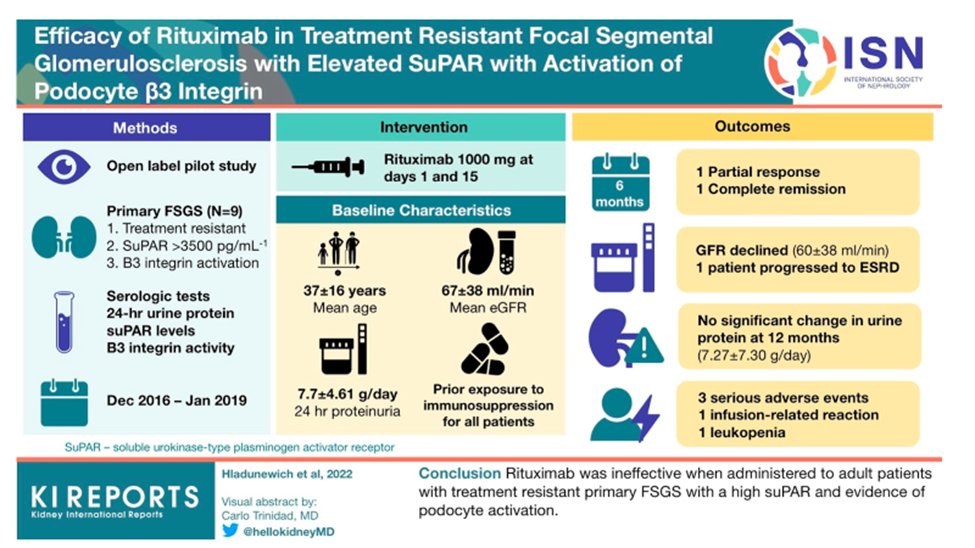
38) #FSGS therapeutic options are limited. Most recently, for instance, #rituximab was shown to be an ineffective therapy
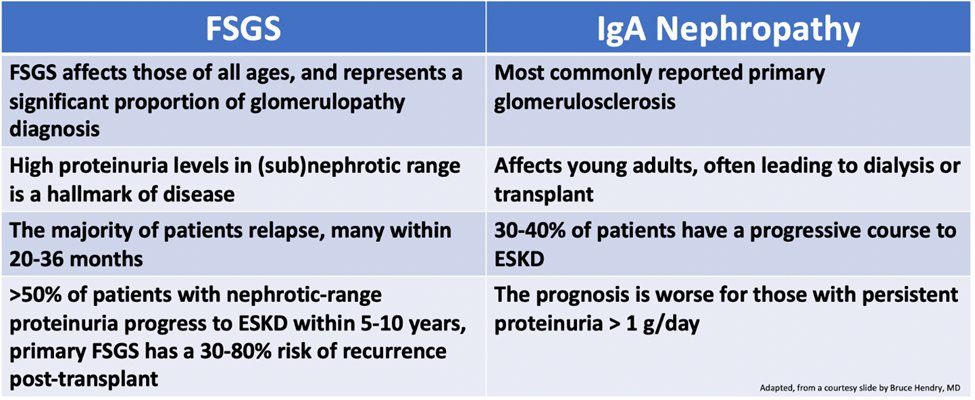
39) #FSGS and #IgAN have some similarities:
40) So, we have reviewed therapeutic options for #IgAN and #FSGS. However, what is the role of the new kid on the block, #sparsentan, a Dual Endothelin Angiotensin Receptor antagonist (#DEARA) in the treatment of these entities?
41) You'll find out TOMORROW, when we'll also wrap up this 🧵and give you your CE/#CME certificate. Meanwhile, what is the most common gene mutation implicated in adult-onset familial FSGS?
a) type 4 collagen gene mutation
b) podocin gene abnormality
c) actin gene abnormality
42) We're back! Today we'll close our discussion of the first-in-class Dual Endothelin Angiotensin Receptor Antagonist (#DEARA) that may be helpful in managing both #FSGS & #IgAN. I am @HecmagsMD & BTW, the answer to yesterday's poll (# 41) is A: a type 4 collagen gene mutation.
43) Early evidence has demonstrated that combined #RAAS blockade and selective ETA inhibition (#sitaxsentan) have a substantial antiproteinuric effect in comparison to nifedipine & placebo
44) Why should we consider use of a dual #ETA and #AT1 receptor antagonist agent, such as sparsentan, in #IgAN & #FSGS? Here is why:
45) Now, let’s go into more details and discuss some of the clinical trials testing the safety and efficacy of sparsentan on IgA nephropathy and #FSGS:
1⃣DUET
2⃣DUPLEX
3⃣PROTECT
4⃣SPARTAN
Let’s dig in:
46) First, let’s talk about the DUET trial, a Ph 2 trial that evaluated efficacy & safety of sparsentan in pts w/ #FSGS. It was an 8-week, #DBRCT of sparsentan vs an active comparator (AT1 receptor blocker, irbesartan) in patients w/ primary FSGS. 🔓
47) In DUET, #sparsentan was well tolerated & generally safe in pts w/ #FSGS. #eGFR remained stable & similar in comparison to #irbesartan, & those treated w/ sparsentan achieved significant ⬇️in #BP & proteinuria compared to irbesartan (44.8% vs 18.5%).
48) Next, the DUPLEX trial, a Ph 3 DBRCT, comparing sparsentan vs irbesartan in pts w/ #FSGS: 🔓
https://www.kireports.org/action/showPdf?pii=S2468-0249%2820%2930003-6. Here are inclusion/exclusion criteria and study design. This trial used the modified FSGS endpoint as a surrogate endpoint.
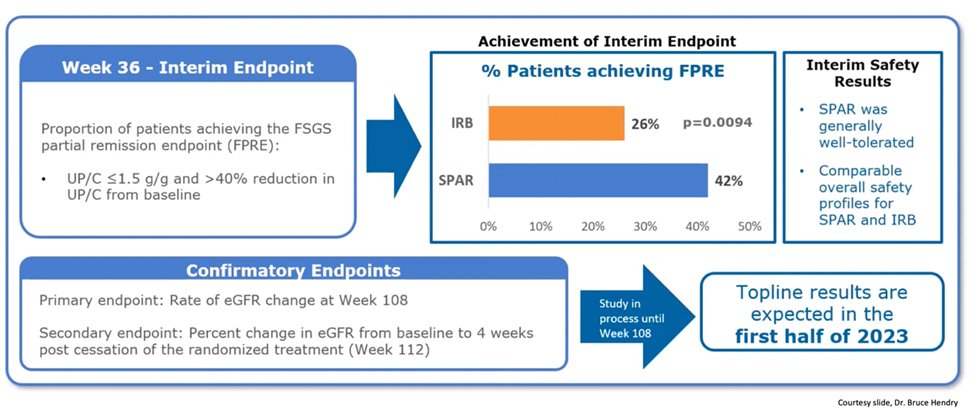
49) Here are the week-36 interim endpoints of the Ph 3 #DUPLEX study in #FSGS, showing positive results. Safety profile was comparable to irbesartan & sparsentan was well tolerated. Full results expected in 2023
50) Next, the Ph 3 #PROTECT study (A study of the Effect and Safety of Sparsentan in the Treatment of Patients with IgA Nephropathy), a global, #DBRCT comparing sparsentan with irbesartan.

51) Primary endpoint in PROTECT is the change from baseline in UPCR in a 24h urine collection at week 36, based on the KHI (Kidney Health Initiative) rec of #proteinuria as being a reasonable surrogate for a drug’s effect on progression to ESKD
🔓https://www.kireports.org/action/showPdf?pii=S2468-0249%2819%2931463-9.
I & E:
52) The PROTECT Study Design is a multicenter, parallel-group, active controlled phase 3 trial, comparing Irbesartan vs sparsentan in #IgAN in pts with > 1 gr of proteinuria on maximal RAAS blockade
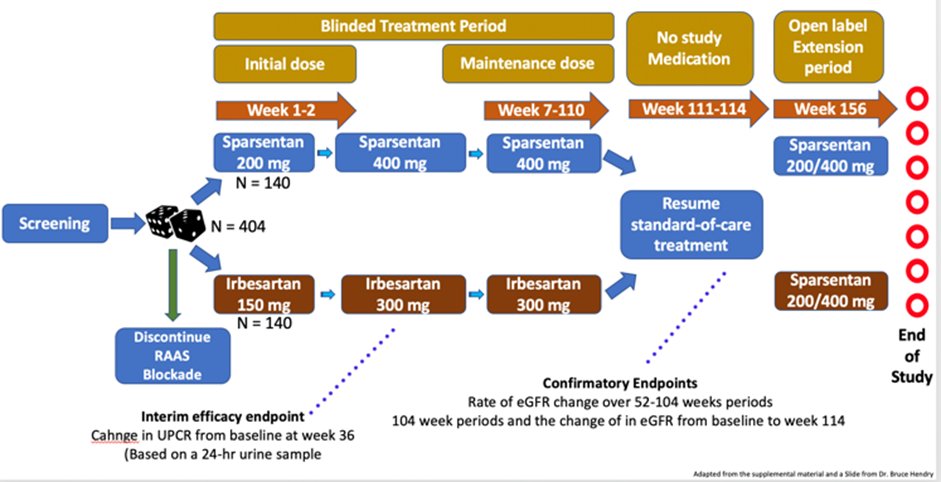
53) In PROTECT, the #eGFR was used as a confirmatory endpoint of treatment effect. eGFR endpoints are statistically more efficient than event-based endpoints for a slowly progressive disease. Traditionally, #ESKD and doubling of serum creatinine have been used in the past.
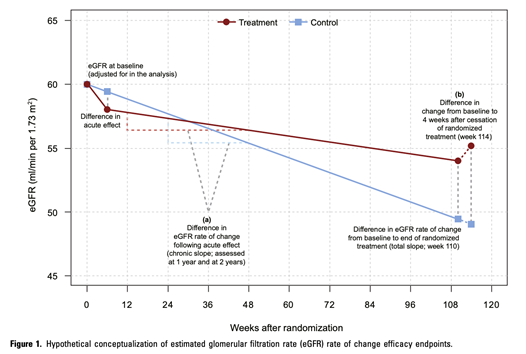
54) These are the week-36 interim data from PROTECT in #IgAN, showing a 49.8% vs 15.1% proteinuria reduction for sparsentan in comparison to irbesartan. Sparsentan was well tolerated. Final results are expected to be published in the second half of 2023.
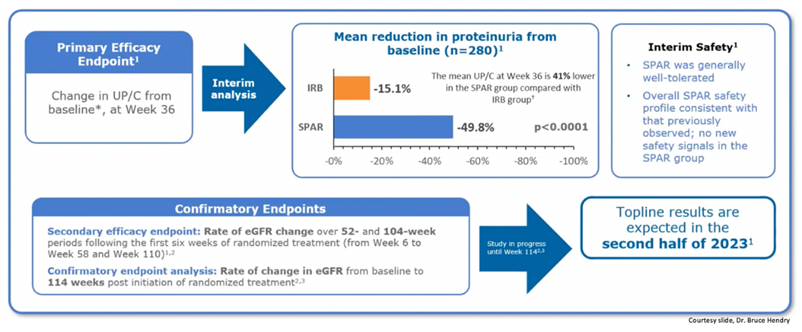
55) And next, we have the #SPARTAN Trial, which will evaluate safety & efficacy of sparsentan in pts newly diagnosed with #IgAN who have not received #RAAS blockade previously. See
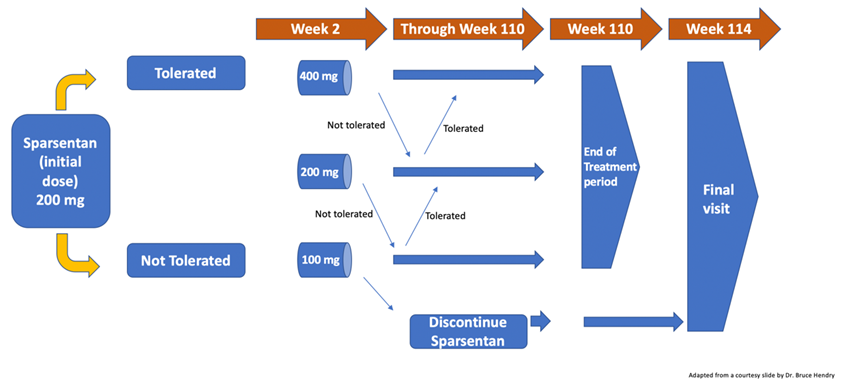
56) SPARTAN is a1⃣center open-label1⃣ cohort study to eval safety & response to sparsentan in incident RAAS blockade-naïve patients w/biopsy-proven IgAN w/ a starting dose of 200 mg/day, up to 400 mg/day if tolerated at wk 2 for a total of 110 wks. Clinical outcomes measures:

57) Here is the SPARTAN trial’s study design. Estimated study completion date is November 2023
58) Finally, the #EPPIK (Evaluating Problematic Proteinuria in Kids) trial, expected to be completed in 2025. This will be a multi-center, open-label, 108-week study in the pediatric population w/ different glomerular diseases (#IgAN, #FSGS, #IgAV, #MCD, #Alport syndrome)
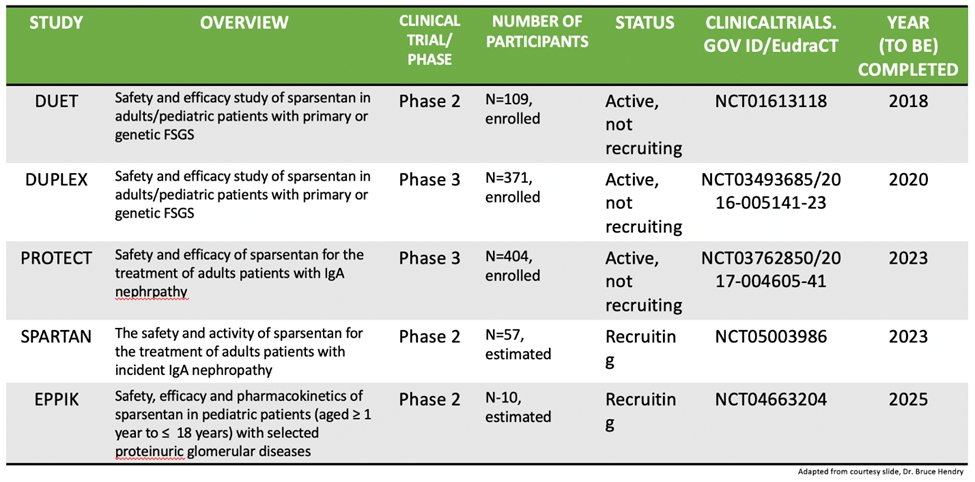
59) Here is a nice table with an overview of the trials we have discussed. Remember, #SPARTAN and #EPPIK are single arm trials.
60) So, are #sparsentan and #atrasentan similar? No. Atrasentan (check SONAR trial https://www.thelancet.com/article/S0140-6736(19)30772-X/fulltext), is a selective Endothelin-A receptor blocker (#ERA), while sparsentan is a Dual Endothelin Angiotensin Receptor Antagonist (#DEARA).
61) ERAs are associated with peripheral #edema and ⬆️incidence of #heartfailure. These side effects have not been seen with DEARAs, so we need to wait for the studies on sparsentan to be published to look at more data.
61) ERAs are associated with peripheral #edema and ⬆️incidence of #heartfailure. These side effects have not been seen with DEARAs, so we need to wait for the studies on sparsentan to be published to look at more data.
63) In conclusion, #sparsentan is a first-in-class, dual ETA and AT1 receptor antagonist (#DEARA) that could be a potential treatment for #IgAN & #FSGS
• Reduces endothelial dysfunction
• Reduces mesangial cell proliferation and fibrosis
• Reduces #podocyte damage
64) As above, there are multiple studies & trials coming out in the next year or so that will answer most if not all of our❓s.
65) And that's it! You just earned 0.75h 🆓CE/#CME credit! Go to https://ckd-ce.com/foundations-DEARA/ and grab your certificate. I am @HecmagsMD and I truly appreciate your joining me. And I suggest you FOLLOW @ckd_ce for more educational programs on renal disease, all by expert authors!
Originally tweeted by @CKD_ce (@ckd_ce) on March 29, 2022.