1) Welcome to a new #accredited #tweetorial regarding evaluation of mechanism of action and potential for therapeutics from combining endothelin type A antagonists and angiotensin II type 1 receptor blockers. Leading us through this material is @didemturgut_ from Turkey 🇹🇷

2) This tweetorial is accredited for 0.5h CE/#CME for #physicians #nurses #NPs #PAs #pharmacists 🇺🇸🇨🇦🇪🇺🇬🇧. Please follow along! Faculty disclosures are at https://ckd-ce.com/disclosures/, and past programs, still available for credit, are at https://ckd-ce.com/. All 🆓!!
3) This educational program is supported by grants from Travere, Bayer, & Otsuka, and is intended for healthcare providers.
4) Let’s start with the historical review of the #endothelin receptor antagonists. In the early 1988 Yanagisawa et al isolated an endothelium-derived 21-residue vasoconstrictor peptide endothelin (#ET), and the journey started. See 🔓
5) In the 21 years since its discovery, ET shown to be one of the most potent #vasoconstrictors known. In mammals, the ET family comprises three endogenous isoforms–ET-1, ET-2 & ET-3–and the receptors that mediate their effects. See https://pubmed.ncbi.nlm.nih.gov/15063086/.
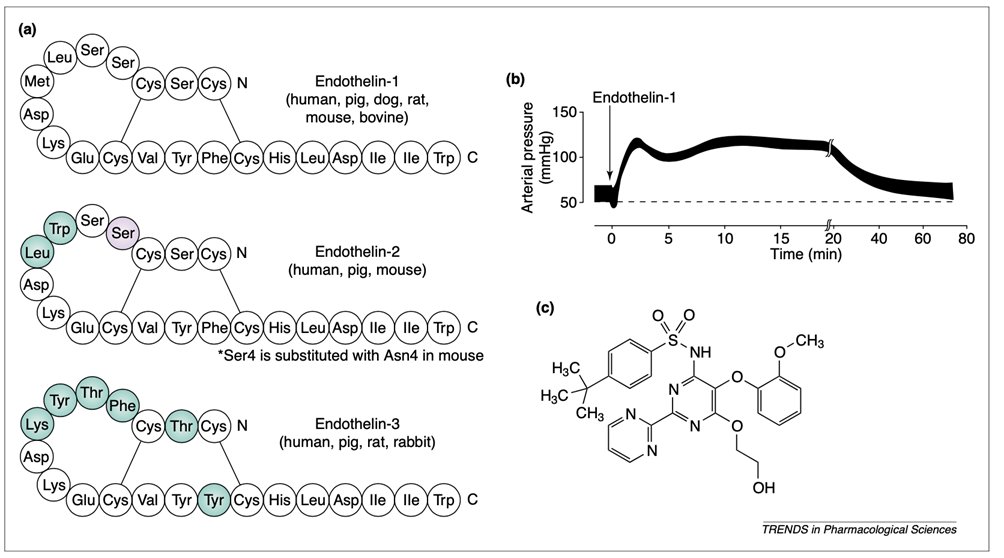
6) ET peptides and ET receptors have been localized to a wide number of tissues and organs. It comes as no surprise that ET has been targeted as a pathophysiological culprit in multiple diseases and/or conditions.
7) There are at least four known ET receptors among the isoforms:, ETA, ETB1, ETB2 and ETC. Explained well in https://www.amazon.com/Medical-Physiology-2e-Updated-PHYSIOLOGY/dp/1437717535. +
8) In human vessels, ETA receptors are mainly located on vascular smooth muscle cells and are mainly responsible for contraction, with ETB receptors being present on endothelial cells lining the vessel wall.
9) ET stimulates proliferation in different cell types (mainly via the ETA receptor) and thought to be co-mitogenic, potentiating the actions of other growth factors. See 🔓https://pubmed.ncbi.nlm.nih.gov/25421754/.
10) So, what about in the kidneys? Which receptor is dominant? Be sure to answer before you scroll ⤵️!
— @CKD_ce (@ckd_ce) May 3, 2022
11) The answer may surprise you!
As per 🔓https://www.seminarsinnephrology.org/article/S0270-9295(15)00028-5/fulltext, Although while measurements of receptors within smooth muscle throughout the renal vasculature show a predominance of ETA, 70% of the ET receptors in both cortex and medulla in human kidney are ETB.
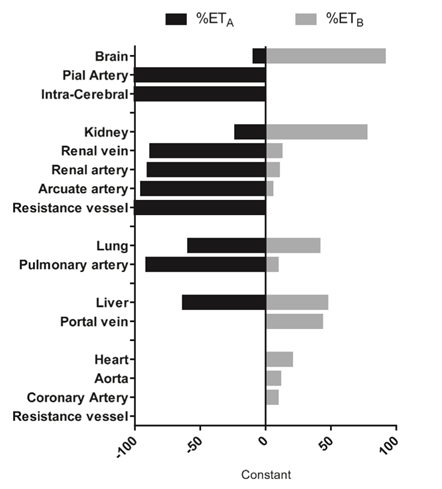
12) But wait–there is another surprise!
#ETA have been shown to be present on human and rat #podocytes. In renal disease, proliferation in mesangial cells, extracellular matrix production, and inflammation are mediated mainly by ETA.
🔓
13) Till tomorrow, think about the potential effects of #ETA and #ETB on the kidney, and potential therapeutic consequences if we control these receptors. Exciting! 👏#FOAMed #medtwitter #nephtwitter @MedTweetorials @TurkNefro @NephrolSocTurk @VijayanMD @priti899 @deniise_am
14) Welcome back! We are providing foundational knowledge on potential for therapeutics that combine antagonists of #endothelin type A & #angiotensin2 type 1 receptors. And YOU are earning CE/#CME! I am @didemturgut_ 🇹🇷 Welcome, @kidneydoc101 @goKDIGO @anace710 @AnandhUrmila
15) Let’s start the day with the drugs antagonizing #ET receptors.
16) They are designed as either ETA-selective antagonists (with an ETA/ETB selectivity ratio > 100) or dual ETA/ETB antagonists. Only a few ETB-selective antagonists have been developed, and these have never reached clinical use. See 🔓
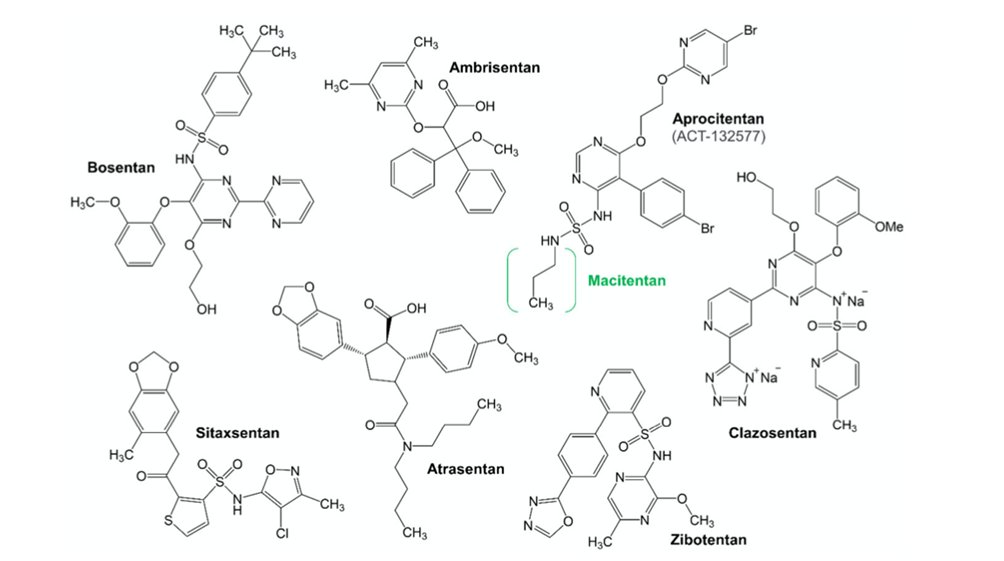
17) Which of the ET receptor antagonists work for kidney diseases, by which receptor blockage?
a) Atrasentan via ETB
b) Sparsentan via ETB
c) Sparsentan via ETA +ARB
d) Sitaxsentan via ETA/ETB
ANSWER BEFORE YOU SCROLL ⤵️!
18) The answer is c. ETA blockers are predominantly studied in the kidney diseases. Again see 🔓
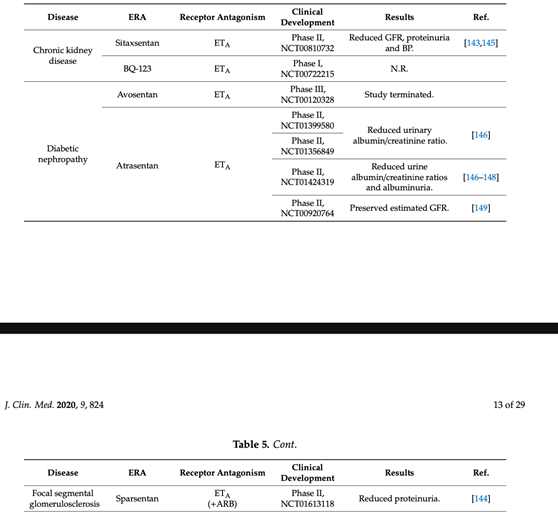
19) Within the kidney, #ETB receptors activation lowers #bloodpressure by promoting #natriuresis & #diuresis by directly inhibiting reabsorption of sodium & water in the #nephron.
20) This suggests that an ETA-selective antagonist could have more beneficial effects than the mixed #ETA and #ETB antagonism in #kidneydiseases. See 🔓

21) Since the kidneys are master chef of the #RAAS, ET becomes the provocative factor for the system. ET-1 ⬆️the formation of ANG II by ⬆️ing activity of angiotensin-converting enzyme.

22) ANG II then activates renal ET-1 production ➡️vasoconstrictor positive feedback loop. 🔓
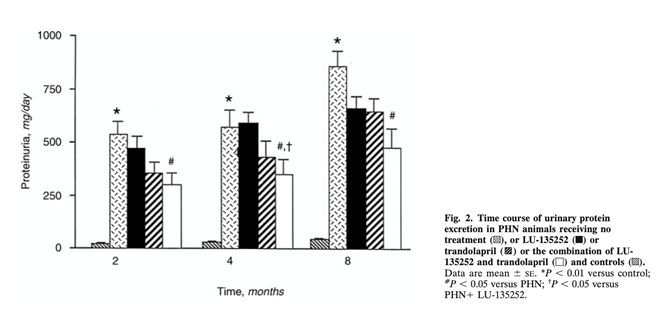
23) Dual therapy for the first time was studied in 1998. ETA receptor antagonist & #trandolopril combination decreased proteinuria prominently in rats with #Heymann nephritis. See 🔓
24) The 1⃣st clinical study with dual therapy was in diabetic nephropathy #DKD. It was relevant that addition of ERAs to a standard nephro-protective therapy with #RAASi ➡️ additional benefits in treatment of progressive diabetic nephropathy. 🔓
25) As the trials continued, some clinical concerns associated with #ERAs came to light. What were the main concerns? Show us what you know before you scroll ⤵️for the correct answer.
— @CKD_ce (@ckd_ce) May 4, 2022
26) The main clinical concern associated with ERAs are dose-related oedemas. But . . . ERAs may induce #headache, #angioedema & #hypotension; sulfonamide-based ERAs may cause #hepatotoxicity. ERAs, similar to RAAS inhibitors are teratogenic. 🔓
27) Tomorrow we'll continue on to ETA receptor antagonists & the DUAL endothelin angiotensin receptor antagonists #DEARAs. COME BACK! For a sneak peek, u can also see (& earn MORE 🆓CE/#CME) at https://ckd-ce.com/foundations-deara/ from @HecmagsMD!
@GoggleDocs @edgarvlermamd @nephondemand
28) Welcome back! I am @didemturgut_ & today we'll wrap up this #tweetorial on the eval of mechanism of action & potential for tx from combining endothelin type A antagonists w/angiotensin II type 1 receptor blockers. So as to clinical trials related to ERAs in #KidneyDisease:
29) Urinary ET-1 excretion, which reflects renal ET-1 production, is ⬆️in every cause of CKD in which it has been studied, including #diabetes, #hypertension, #glomerulonephritis, #PCKD, & others. See https://pubmed.ncbi.nlm.nih.gov/9002526/.
30) The effects of acute intravenous administration of ERAs on #CKD patients have investigated in a series of very nice studies.
31) For example: after administration of BQ-123 (ETA receptor antagonist), the⬇️ in proteinuria & pulse wave velocity was greater in those subjects receiving dual ACE-I/ARB treatment than in those on ACE-I alone. Patients were nondiabetic #CKD patients.
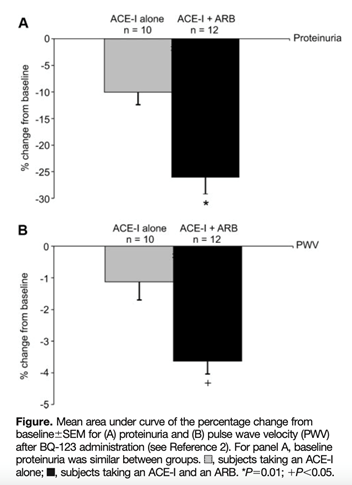
32) Data suggesting that ETA blockade in #CKD may be most effective in the setting of maximal #RAAS blockade provided insight into the use of dual therapies to be used immediately in trials with #CKD or #DKD.
33) In the ASCEND study, avosentan decreased ACR after a median follow-up of 4 months. Patients were on optimal RAS blockade. However the trial was prematurely terminated due to adverse cardiovascular events in the avosentan groups. See 🔓https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2831858/.
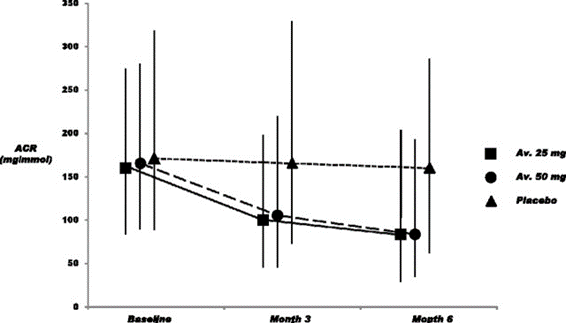
34) It was hypothesized that at the higher doses, avosentan may act on the ETB receptor to promote additional fluid retention. Given the failure of the ASCEND trial, subsequent trials examining ERAs in kidney diseases were undertaken w/very careful ERA dose & patient selection.
35) In the RADAR study, a subsequent Ph 2b trial of 211 patients with #T2D on #RAASi with #eGFR 30–75 ml/min/1.73m2 & #UACR 300–3500 mg/g, atrasentan (0.75 or 1.25 mg/day) reduced UACR over 12 weeks by ~ 36% at either dose. See 🔓https://pubmed.ncbi.nlm.nih.gov/24722445/.
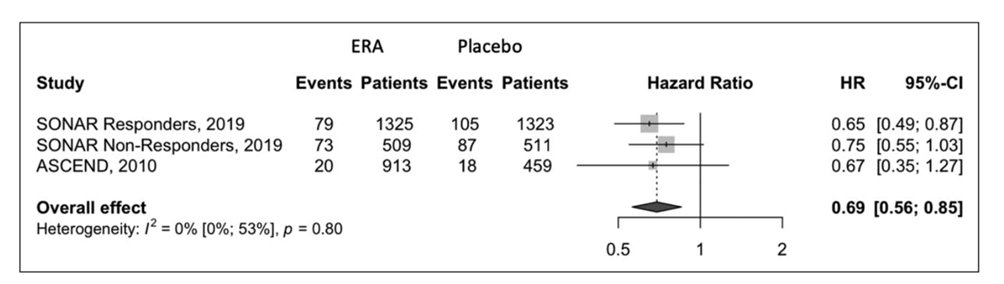
36) Encouraged by RADAR, the Ph 3 Study of Diabetic Nephropathy with Atrasentan (SONAR, https://pubmed.ncbi.nlm.nih.gov/30995972/) was conducted in adults with T2D, eGFR 25–75 ml/min/1.73 m2, & UACR 300–5000 mg/g who were receiving maximal tolerated dose of RAASi.
37) The important lessons from #SONAR were:
👉 #Atrasentan significantly reduced renal events in type 2 diabetes.
👉 Atrasentan did not alter composite major cardiovascular outcomes
👉 It did reduce nonfatal stroke
38) The idea of combining structural elements of RAS (AT1) & ETA antagonists➡️dual endothelin angiotensin receptor antagonist (#DEARA). #Sparsentan is a first-in-class, orally active DEARA w/similar high affinity to both receptors.
39) Its mechanism of action is based on structural similarities with #irbesartan and some ETA receptor antagonists. See 🔓https://pubmed.ncbi.nlm.nih.gov/27009050/.
40) So based on that #MOA, for what diseases might #DEARAs potentially work?
— @CKD_ce (@ckd_ce) May 5, 2022
41) It's ALL! *7⃣* Ph 1 clinical studies in healthy volunteers & 2 Ph 2 studies in pts w/essential #hypertension but not yet published. Focus was not on proteinuria but on #HTN, where effects stronger than those of irbersartan. https://pubmed.ncbi.nlm.nih.gov/14718594/; 🔓
42) Subsequently, clinical trials have been conducted to assess efficacy & safety of #sparsentan in different #glomerulopathies. See again
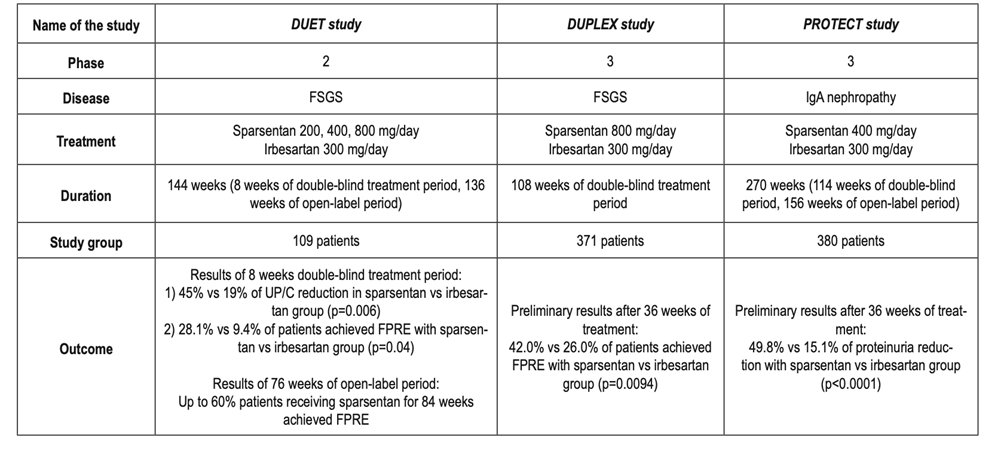
43) And as u learned from @HecmagsMD while u earned CE/#CME at https://ckd-ce.com/foundations-deara/, the journey is only beginning. New studies of #sparsentan are currently enrolling. #SPARTAN (https://clinicaltrials.gov/ct2/show/NCT04663204): pts w/bx-proven #IgAN but no #RAASi will be treated with sparsentan . . .
44) … for 110 weeks to assess its #nephroprotective potential. In #PROTECT (https://clinicaltrials.gov/ct2/show/NCT03762850), same goal but by comparing long-term (2 yrs) impact of sparsentan vs an #ARB. And there's a pediatric study, https://clinicaltrials.gov/ct2/show/NCT05003986 …
45) … with selected #proteinuric #glomerular diseases such as #FSGS, Minimal Change Disease #MCD, #IgAN, Immunoglobulin A-Associated #Vasculitis, & #Alport Syndrome
46) The limited current treatment options in kidney diseases indicate a compelling need to develop an effective and safe nephroprotective therapy. As preliminary results from clinical trials are encouraging, sparsentan could become one of these therapies.
47) And so you have made it! 🆓CE/#CME! #Physicians #pharmacists #nurses #PAs: go to https://ckd-ce.com/foundations_deara2/ and claim your credit! I am @didemturgut_ . Follow @ckd_ce (& @cardiomet_CE) for more #tweetorials! #medtwittter #nephtwitter #FOAMed
Originally tweeted by @CKD_ce (@ckd_ce) on May 3, 2022.