Join us tomorrow for the launch of a new accredited tweetorial – a case-based program on the assessment and management of patients with IgA #nephropathy (#IgAN). Free CE/#CME for #physicians, #nurses, #pharmacists! Expert author none other than @IgAN_JBarratt. FOLLOW US . . .
. . . and tell your colleagues to join you here on @ckd_ce, your ONLY source for accredited #tweetorials in the #CKD space! #nephtwitter #nephjc @nephondemand @Nephro_Sparks @edgarvlermamd @ChristosArgyrop @goKDIGO @nicklimd @CKJsocial @ERAkidney @kidney_boy @kidneypathology
1) Welcome to our accredited #tweetorial on optimal contemporary mgt of #IgAN. Earn 0.5h CME/CE credit #physicians #nurses #nursepractitioners #physicianassistants by following this thread. I am @IgAN_JBarratt. @MedTweetorials #nephtwitter #nephjc #FOAMed #MedEd

2) The @ckd_ce program is supported by educational grants from Travere, Bayer, and Otsuka. This program is intended for healthcare providers. Faculty disclosures can be found at https://ckd-ce.com/disclosures/. Follow us here for #accredited #tweetorials by #experts in #nephrology!
3) First, a case: caucasian 23M referred for #CKD evaluation after an abnormal urinalysis identified in routine eval. No Meds. BMI 29 kg/m2. BP 141/85 mmHg. Urinalysis blood ++, protein ++. Key Labs: eGFR 68ml/min & UPCR 293mg/mmol. Kidney biopsy shows #IgAN . . .
4) . . . with an Oxford score of M1, E0, S1, T0, C0. See info on Oxford score at https://pubmed.ncbi.nlm.nih.gov/34556256/ and use the handy calculator at https://qxmd.com/calculate/calculator_499/international-igan-prediction-tool-adults. Also see discussion of the score (and earn MORE CE/#CME at https://ckd-ce.com/igan-kdigo/, thanks to @edgarvlermamd)
5) The risk of a 50% decline in #eGFR or #ESKD can be calculated out to 5 years at the time of biopsy using the International IgAN Risk Prediction Tool using the data available within 6 months of the kidney biopsy. Quantifying the risk of progression at the time of diagnosis …
6) … facilitates shared decision making with the patient when discussing treatment options and future plans. See also https://pubmed.ncbi.nlm.nih.gov/30980653/, https://pubmed.ncbi.nlm.nih.gov/31550359/, https://pubmed.ncbi.nlm.nih.gov/32464215/.
7) Risk Factors included in the International IgAN Risk Prediction Tool include: eGFR, BP, UPCR, age, race, prior/current treatment and Oxford score and for this patient the calculated risk of a 50% decline in eGFR or ESKD at 5 years is 14.32%.
8) The Risk Prediction Tool is now being developed to allow its use at 1 & 2yr post-biopsy. In addition, new studies are evaluating whether addition of novel biomarker data can⬆️prognostic accuracy of the Risk Prediction Tool. (https://pubmed.ncbi.nlm.nih.gov/33417998/, https://pubmed.ncbi.nlm.nih.gov/34386667/)
9) Additional clinicopathological factors reported to ⬆️risk of progressive kidney function⬇️in #IgAN include⬆️BMI #smoking & extent of complement activation in the kidney biopsy
https://pubmed.ncbi.nlm.nih.gov/22350469/, https://pubmed.ncbi.nlm.nih.gov/32878271/,
https://pubmed.ncbi.nlm.nih.gov/20471735/,
10) There are still no validated prognostic serum or urine biomarkers for IgAN other than eGFR and #proteinuria. See
11) Meanwhile, the immediate focus for treatment in this case is to deliver comprehensive supportive care which should include (https://pubmed.ncbi.nlm.nih.gov/34495361)
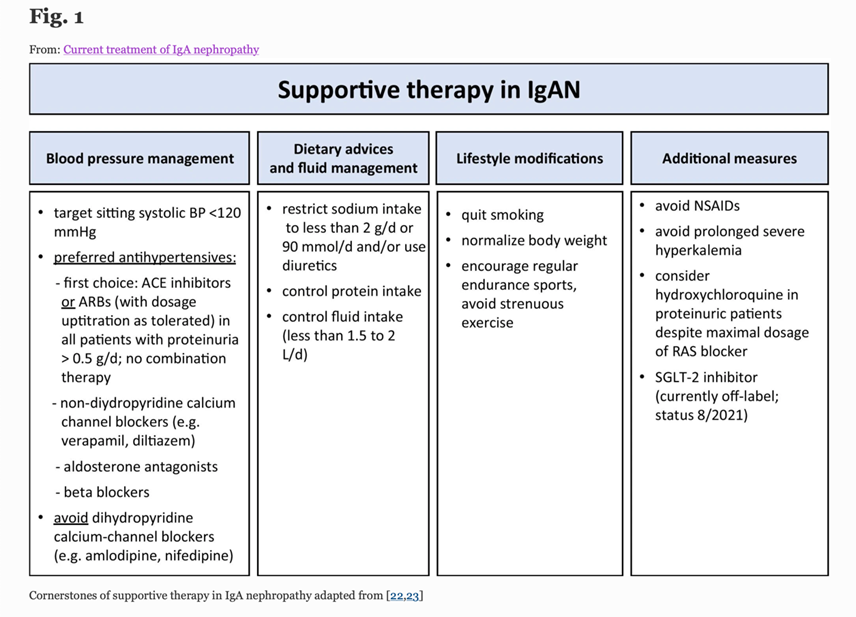
12) Response to optimised supportive care should be assessed once the patient has been on maximal tolerated dose of #ACE inhibitor or #ARB for at least 90d.
13) At this point persistent proteinuria >1g/24h identifies patients at high risk of progressive CKD who may benefit from additional therapy.
(https://pubmed.ncbi.nlm.nih.gov/17978307)
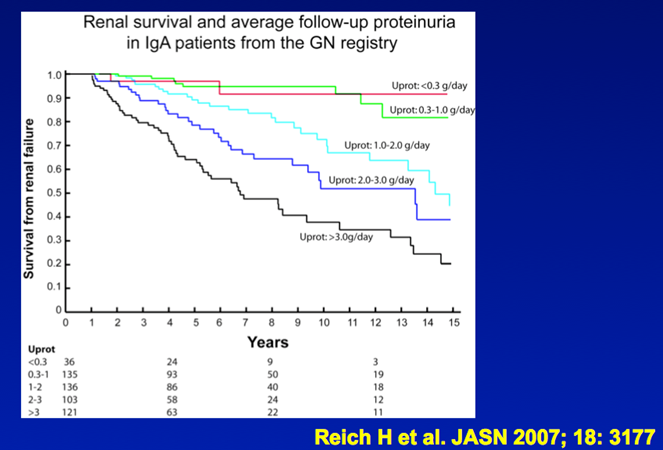
14) The STOP-IGAN study included 337 adults with IgAN and proteinuria > 0.75g/d, plus #hypertension and/or eGFR < 90 ml/min/1.73m2). Patients were randomised to continue receiving supportive care or supportive care plus #immunosuppressive therapy.
15) #Renal outcomes have now been followed out to 10 years. There was no difference in the frequency of ESKD or eGFR decline of >40% primary outcome between the supportive care and additional immunosuppression groups.
16) Notably 50% of patients in each arm experienced a >40%⬇️in eGFR or ESKD at 8yr highlighting the limitations of a supportive care-only approach & available immunosuppression regimens. See https://pubmed.ncbi.nlm.nih.gov/26630142/,
https://pubmed.ncbi.nlm.nih.gov/29042456/,
https://pubmed.ncbi.nlm.nih.gov/32450154/.
17a) After 3 months of optimized supportive care you review the 23M with IgAN who is now taking ramipril 10mg od. BMI 26 kg/m2. BP 117/73 mmHg. Urinalysis blood ++, protein ++. Key Labs: eGFR 60ml/min & UPCR 171mg/mmol. What would you do next for this patient? (Choices below)
17b)
A. Add an angiotensin receptor blocker
B. Add oral prednisolone
C. Add rituximab
D. Offer opportunity to join a clinical trial
18) Mark your answer and return tomorrow for more on this case and more education! @kidneydoc101 #nepharchive #nephtwitter @ssfarouk @Neph_SIM @cheekaycheung @dr_denby @geddescc @Kidney_Research @kidneycareuk @MVankalakunti @RenalFellowNtwk @GlomCon @NephRodby
19) Welcome back! We are talking about the assessment & management of patients with IgA #nephropathy (#IgAN) and we are being led to knowledge and CE/#CME credit 🇺🇸🇨🇦🇪🇺🇬🇧 by @IgAN_JBarratt. Follow us for great programs like this!
@BradRovin @cheekaycheung @geddescc @Renaltubules
20) Re yesterday’s poll, the updated KDIGO 2021 Guidelines suggest the first course of action should be to offer the patient an opportunity to participate in a clinical trial if one is available🔓
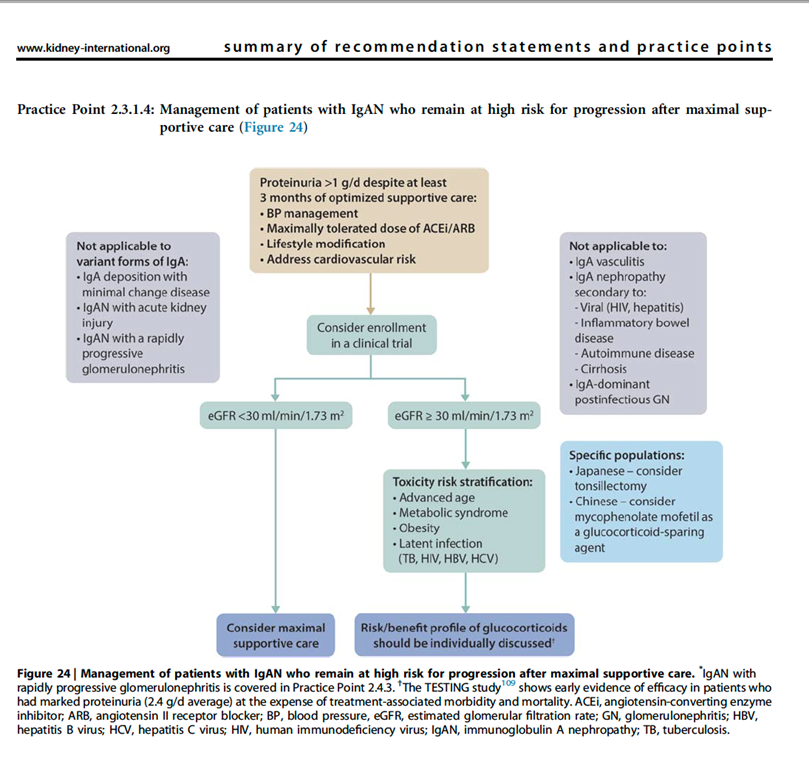
21) Currently enrolling clinical trials require patients to be on maximal supportive care for a minimum of 90 days before patients are eligible for screening.
22) In most studies #UPCR will need to be above 1g/24h measured on a 24h collection as this has traditionally been accepted as defining an increased risk of progression in IgAN.
23) New data suggest that a more precise way to identify patients who may benefit from therapy above supportive care either in the context of a clinical trial or using available treatments is to use the IgAN risk prediction tool.
See
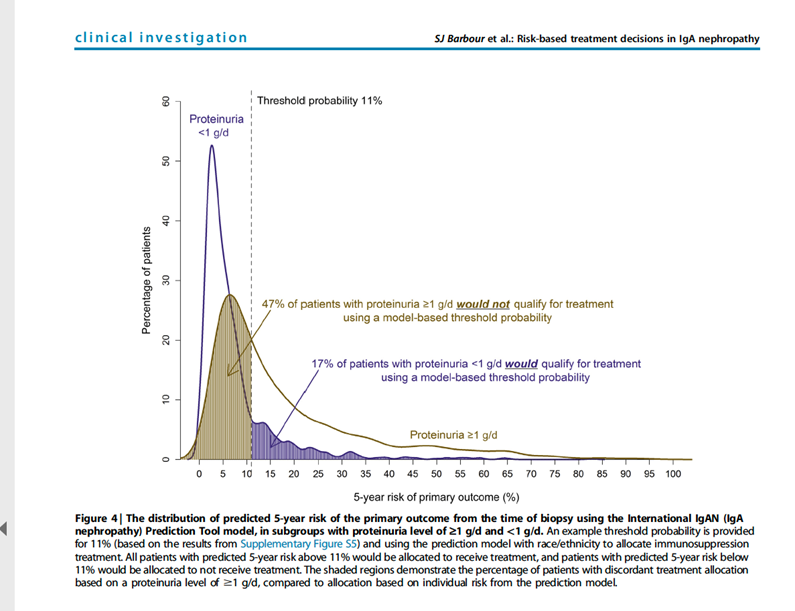
24) By the way . . . all of the following are true of that International IgA prediction tool except:
a) uses MEST-C score
b) predicts 5-year prognosis at the time of biopsy
c) details which therapeutic regimen will work the best
d) I'm in the dark – please help!
25) The answer is c; the International IgA prediction tool doesn’t predict therapeutic response—it’s prognostic only.
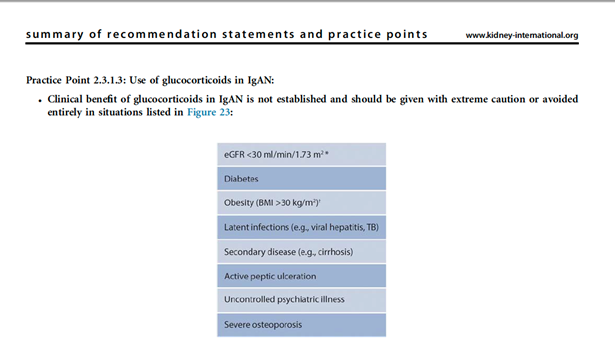
26) If it is not possible to recruit to a clinical trial then consider systemic #corticosteroids once a detailed risk:benefit assessment has been undertaken.
27) It is important that the patient is made aware of the risks of taking systemic corticosteroids, and in fact corticosteroids may be contraindicated in specific situations
🔓https://kidney360.asnjournals.org/content/2/7/1081
🔓https://kidney360.asnjournals.org/content/2/7/1078
🔓
28) Data from the recently completed Low-dose TESTING Study (https://clinicaltrials.gov/ct2/show/NCT01560052) has now been presented at #ASN21 and we await the full manuscript. Importantly this is not a trial of low dose corticosteroids:
29) … a typical 70kg pt would have received 35-40mg/d prednisolone in this protocol & there were <10% Caucasian study participants.
A key recommendation in the updated @goKDIGO 2021 #IgAN Guideline is to evaluate therapeutic strategies to ⬇️or avoid systemic glucocorticoids
30) So what are the emerging alternatives to steroids? Let's explore new therapies currently being evaluated in #IgAN. These broadly fall into 2⃣therapeutic categories:
a) augmentation of supportive care
b) immunomodulatory therapies
31) As data emerge from these studies the @goKDIGO GD guidelines will be reviewed and updated updated on an annual basis.
See a prior evaluation of current @goKDIGO guidelines in this space from @edgarvlermamd, still available for credit, at https://ckd-ce.com/igan-kdigo/.
32) Additional supportive care approaches that may prove useful include endothelin receptor antagonism (#ERA) & #SGLT2i.
33) Endothelin-1, through activation of endothelin A receptors, has been strongly implicated in renal cell injury, proteinuria, inflammation, & fibrosis➡️#CKD. 🔓
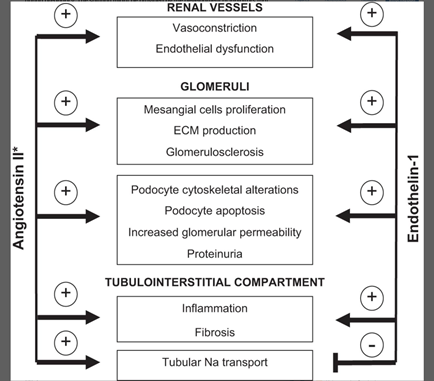
34) Endothelin receptor antagonists (ERAs) ameliorate or even reverse renal injury and/or fibrosis in experimental models of CKD (🔓https://journals.physiology.org/doi/full/10.1152/ajpregu.00425.2015).
35) Clinical trials have shown a substantial antiproteinuric effect of ERAs in diabetic & nondiabetic CKD patients even on top of maximal renin-angiotensin system blockade (https://pubmed.ncbi.nlm.nih.gov/33990507/).
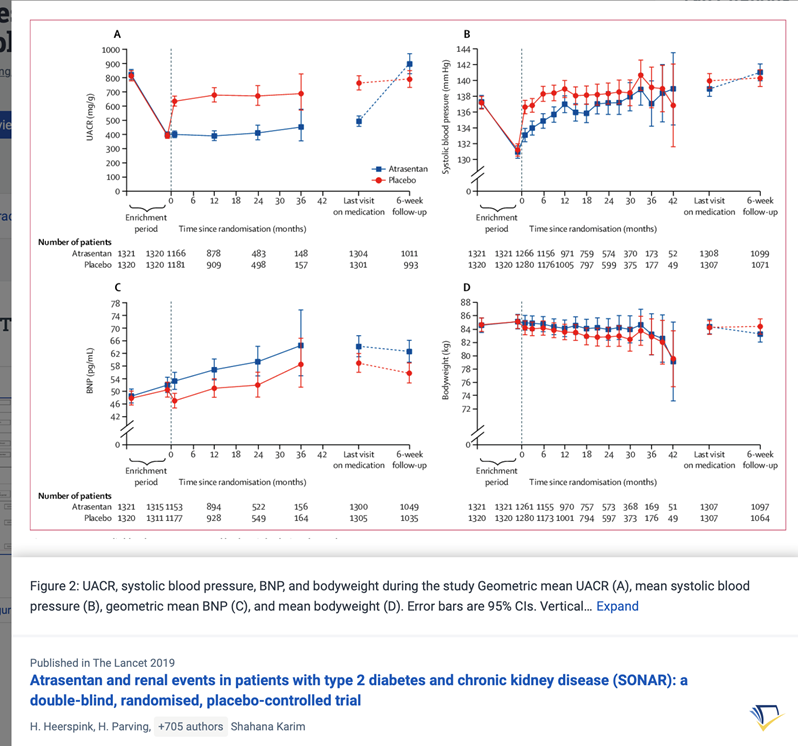
36) ERAs are currently being evaluated in the PROTECT (🔓https://pubmed.ncbi.nlm.nih.gov/31891005/ & https://www.clinicaltrials.gov/ct2/show/NCT03762850; #sparsentan recruitment closed) and ALIGN (https://clinicaltrials.gov/ct2/show/NCT04573478, #atrasentan, still recruiting) Ph3 studies.
37) 9 month #UPCR data in the PROTECT trial shows ERA ➡️50% ⬇️in mean UPCR compared to 15% ⬇️with placebo.
This antiproteinuric effect of #ERA is consistent with earlier studies of ERA in #FSGS (🔓https://jasn.asnjournals.org/content/29/11/2745) and DKD (see https://pubmed.ncbi.nlm.nih.gov/30995972/).
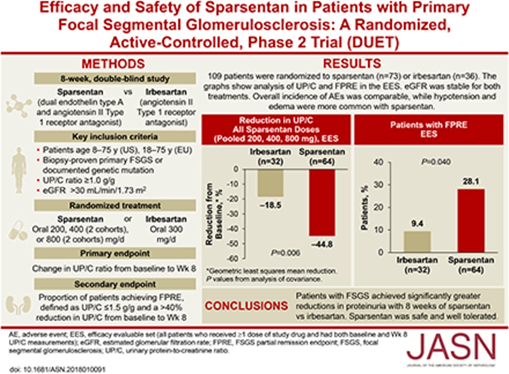
38) #SGLT2i's improve outcomes in diabetic and nondiabetic #kidneydisease (🔓https://www.nejm.org/doi/full/10.1056/NEJMoa2024816), and a prespecified analysis of the DAPA-CKD trial has reported a ⬇️risk of #CKD progression with a favorable safety profile in #IgAN (🔓https://pubmed.ncbi.nlm.nih.gov/33878338/).
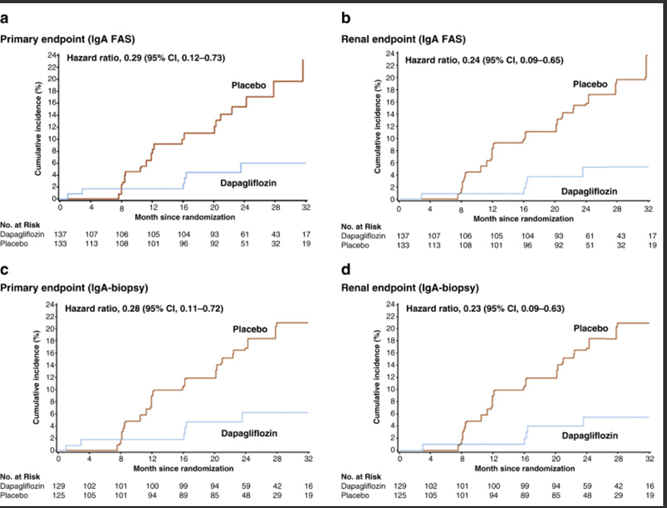
39) There are some important features in the #DAPA-CKD trial design & # IgAN patient population in this subgroup analysis (https://pubmed.ncbi.nlm.nih.gov/33878337/) that mean further data are required to be confident that #SGLT2i add value above optimised supportive care in IgAN.
40) The #EMPA-KIDNEY study (https://clinicaltrials.gov/ct2/show/NCT03594110) may provide these.
Look out tomorrow for a summary of new immune-directed approaches currently being tested in #IgAN, and for your link to🆓 CE/#CME for #physician #physicianassociate #nurse #NP #Pharmacist from @academiccme !
41) Welcome back! You are just a few 🖱️clicks away from your free CE/#CME credit! Expert #nephrologist @IgAN_JBarratt is teaching us all about current and emerging management of #IgAN. Nods to
@didemturgut_ @dguerrot @SWadhwaniMD @ssfarouk @arvindcanchi @m_furlano @JZRenalPath
42) With a better understanding of the pathogenesis of #IgAN it has become possible to design and deliver more targeted treatment to different components of the pathogenic cascade (https://pubmed.ncbi.nlm.nih.gov/30665568/).
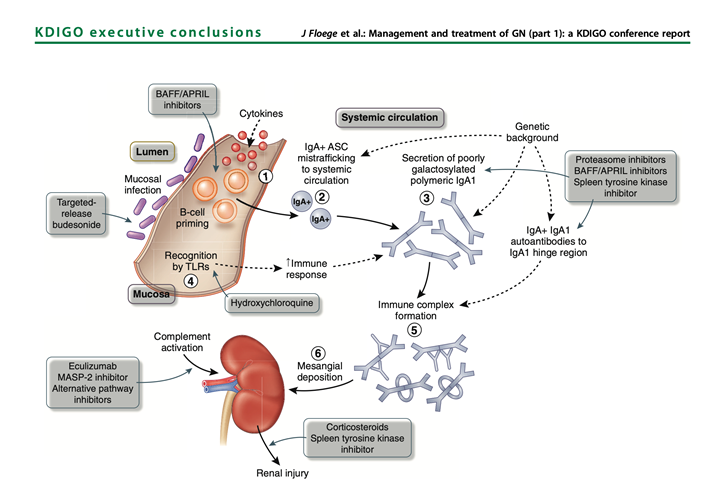
43) A new targeted release formulation of the corticosteroid #budesonide (Nefecon/Tarpyeo), designed to deliver budesonide to the mucosal immune system in the terminal ileum, has recently received accelerated approval by #FDA for use in pts with IgAN at high risk of progression.
44) Patients in the NefIgArd study remain under follow-up to measure the impact of budesonide on rate of decline of #eGFR at 2 years (https://pubmed.ncbi.nlm.nih.gov/28363480/ & 🔓https://pubmed.ncbi.nlm.nih.gov/33102954/). The trial manuscript is awaited.
45) #Hydroxychloroquine has been evaluated in a small #RCT in China which reported a significant⬇️ proteinuria (https://pubmed.ncbi.nlm.nih.gov/30922594/). These findings must be replicated in a larger cohort and include a Caucasian population.
46) A number of drugs targeting the BAFF/APRIL axis are in Ph 2 at present (https://clinicaltrials.gov/ct2/show/NCT04287985; https://clinicaltrials.gov/ct2/show/NCT03945318; https://clinicaltrials.gov/ct2/show/NCT04716231) with early reports suggesting a significant lowering effect on galactose deficient IgA & #proteinuria (https://pubmed.ncbi.nlm.nih.gov/31027890/).
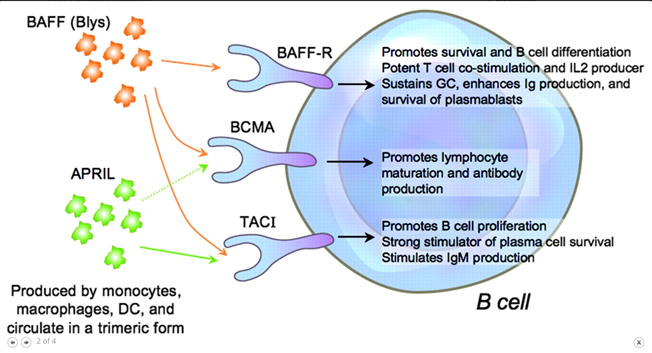
47) Inhibition of the #complement cascade is another area of intense study. Inhibitors of the alternative (LNP023/iptacopan: https://clinicaltrials.gov/ct2/show/NCT04578834), lectin (OMS721/narsoplimab:🔓https://www.kireports.org/article/S2468-0249(20)31441-8/fulltext & https://clinicaltrials.gov/ct2/show/NCT03608033) & of the final common pathway . . .
48) … (APL-2/pegcetacoplan [https://clinicaltrials.gov/ct2/show/NCT03453619], ravulizumab [https://clinicaltrials.gov/ct2/show/NCT04564339] & cemdisiran [https://clinicaltrials.gov/ct2/show/NCT03841448]) of complement activation are currently being investigated in #IgAN (see 🔓https://pubmed.ncbi.nlm.nih.gov/30941137/).
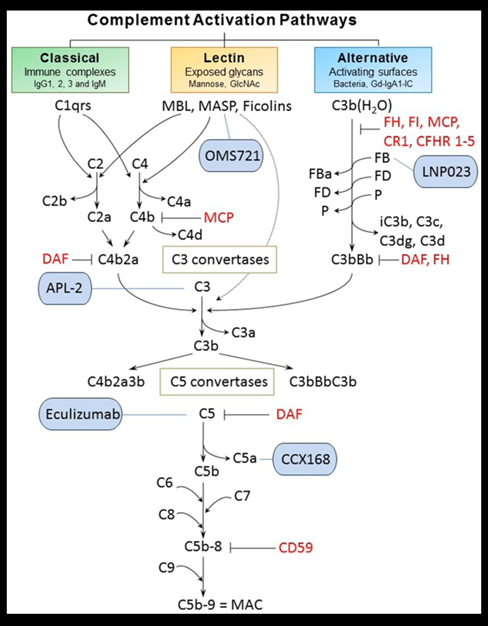
49) Interim data presented for both narsoplimab and iptacopan support complement inhibition as an approach likely to improve outcomes for IgAN patients at high risk of progressive disease.
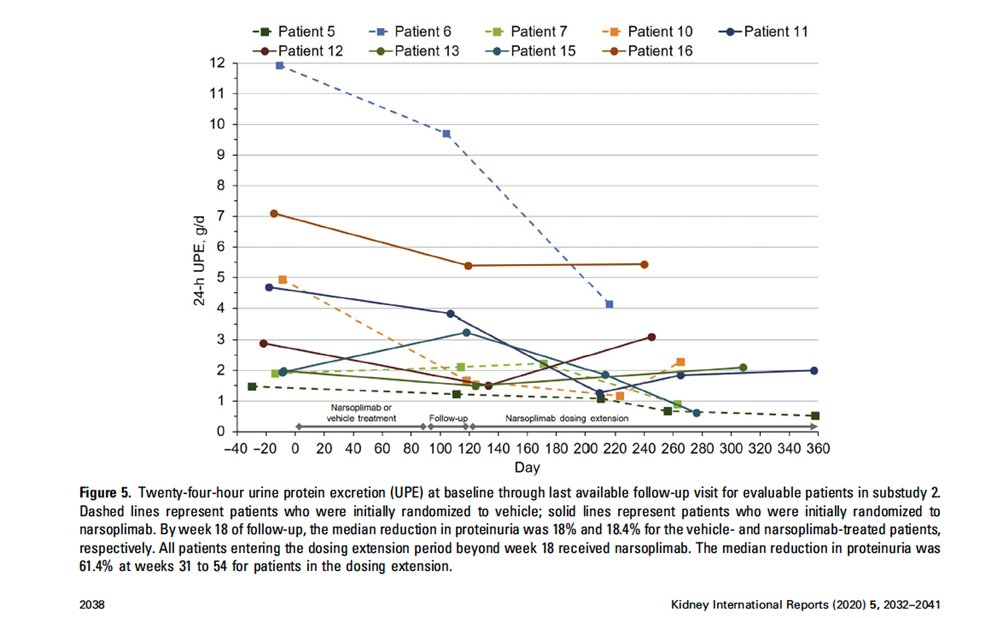
50) The challenge now is to think about how we might use all of these new drugs to manage the 23 year old we discussed earlier………..
51) So many❓come to mind: Should we start designing induction and maintenance of remission drug regimes in IgAN? Which combinations of therapies might work best together? When might we switch from one therapy to another? Should we biopsy patients more often . . .
52) … to assess disease activity and tailor treatment to activity? Which biomarkers can we use to guide treatment and monitor treatment response?
All of these questions will be the focus of clinical research for the next 5-10 years and we will need your help to answer them!
53) And so you made it! You just learned SO MUCH and you also earned 0.75h CE/#CME credit. Please go to http://www.ckd-ce.com/IgAN3 and claim it! I am @IgAN_JBarratt and I invite you to follow @ckd_ce for more great programs and 🆓credit!
Originally tweeted by @CKD_ce (@ckd_ce) on February 28, 2022.